Use a 24 in front of F2 since 24 x 2 also equals 48. WebView Copy of Module Six Lesson One Guided Notes.pdf from SCI NA at Washington County High School. H2O2 is a completely different substance from H2O. Here's another method. Since the lowest common denominator is 4, each of the variables must be multiplied by 4. So our new function which I have called addToMatrix has the following parameters: Before we begin writing our new function, we want to create two global lists which I will call elementList and elementMatrix. 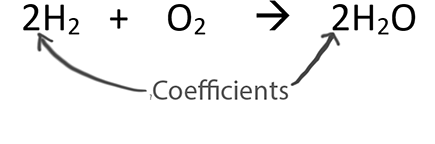 Get smarter at building your thing. For example, when the coefficient 3 is assigned to the CO. Once all the individual elements are balanced, the total number of atoms of each element on the reactant and product side are compared once again. We have to get both balanced. What does the symbol in a chemical equation mean? You never change subscripts. One reason that our program is so strong is that our . In order to illustrate this method, the combustion reaction between propane and oxygen is taken as an example. Therefore, the following relations can be made to obtain the equation for oxygen: Therefore, the equation for oxygen can be written as: The equations for each element are listed together to form a system of equations. All the atoms on the reactant side of and equation are also on the product side. Subscripts: The small numbers written to the right of the atoms. See that 10 is the least-common multiple between 2 and 5: 2) Multiply through by 2 to clear the fraction: Example #16: KFe3AlSi3O10(OH)2 + Cu + O2 + H2S ---> KAlSi3O8 + CuFeS2 + H2O.
Get smarter at building your thing. For example, when the coefficient 3 is assigned to the CO. Once all the individual elements are balanced, the total number of atoms of each element on the reactant and product side are compared once again. We have to get both balanced. What does the symbol in a chemical equation mean? You never change subscripts. One reason that our program is so strong is that our . In order to illustrate this method, the combustion reaction between propane and oxygen is taken as an example. Therefore, the following relations can be made to obtain the equation for oxygen: Therefore, the equation for oxygen can be written as: The equations for each element are listed together to form a system of equations. All the atoms on the reactant side of and equation are also on the product side. Subscripts: The small numbers written to the right of the atoms. See that 10 is the least-common multiple between 2 and 5: 2) Multiply through by 2 to clear the fraction: Example #16: KFe3AlSi3O10(OH)2 + Cu + O2 + H2S ---> KAlSi3O8 + CuFeS2 + H2O.  (d) 3Ca(NO3)2 (just the oxygens) ---> There are 18. Procedure. To breakdown the regex, the outermost parenthesis(near the single quotes) indicate that that is our capture group and it is what we want to keep. 3) Examine the situation with hydrogen and oxygen and discover they are both balanced with 8 of each. The unbalanced chemical equation is: balancing the number of oxygen and hydrogen atoms first and then balancing the number of sodium atoms, the balanced chemical equation is found to be: Put your understanding of this concept to test by answering a few MCQs. The moment of inertia of the rod and bracket about the vertical axis of rotation is 0.30kgm20.30 \mathrm{~kg} \cdot \mathrm{m}^20.30kgm2 and the centroidal moment of inertia of the tube about a vertical axis is 0.0025kgm20.0025 \mathrm{~kg} \cdot \mathrm{m}^20.0025kgm2. Group of answer choices, Name the most active metal in the reaction below: How many grams of oxygen are formed when 5.00 g of dinitrogen monoxide decomposes? Count the number of atoms of each element, compound or ion in the reactants and products. Look for it in the solved examples. Determine the number of sulfur atoms in 27.1 g of molecular sulfur (S8). Im going to pass to it: Before writing this function, we are going to remove our print statements and modify our previous function compoundDecipher to call it as follows: we will start off by separating out the elements and numbers using a regex and looping through every element with a while loop. There is also a slower but more systematic approach using linear algebra. This happens fairly often at the end of a balancing sequence, when the placement of one coefficient balances two different elements at the same time. Why is it necessary for nitrate to be in parentheses but it is not necessary for sulfate? Whenever The charge of the ions must balance. 3. Before we go any further, id like to point out that the majority of the programming will be with regards to populating a matrix with the quantities of our chemical equations and we will be importing a library to handle solving the matrix. Step 2: Determining This problem is interesting because you focused on the oxygens first. The reaction C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l) is best classified as a: On a molecular level, what do the coefficients mean in the following chemical equation: N2 (g) + 3 H2 (g) 2 NH3 (g)? I'll use a fraction to balance it: 4) Multiply through to clear the fraction: You may have protested at the 52 used with the Si. 1) Hint: think about what the least common multiple is between 2 and 3. To start off we will be taking user input using Pythons built in input() function. You then multiply through by 2 to get the whole number set of coefficients, the 2, 2, 3 just above. The cookies is used to store the user consent for the cookies in the category "Necessary". watching videos 1 and 2, the students should answer the attached If you want to play around with regular expressions you can view this one here. Therefore, there must be 3 O2 molecules that yield 2 Al2O3 atoms. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. This system of equations can have multiple solutions, but the solution with minimal values of the variables is required. Therefore, the system of equations is transformed as follows: Substituting the values of a,c, and d in the equation 6a + 2b = 2c + d, the value of b can be obtained as follows: It is important to note that these equations must be solved in a manner that each variable is a positive integer. MITs Alan , In 2020, as a response to the disruption caused by COVID-19, the College Board modified the AP exams so they were shorter, administered online, covered less material, and had a different format than previous tests. All chemical calculations you will see in other units must be done with a balanced equation. Step 3 is repeated until all the number of atoms of the reacting elements are equal on the reactant and product side. WebAdding a coefficient of 2 to the H 2 O would balance the eqn.
(d) 3Ca(NO3)2 (just the oxygens) ---> There are 18. Procedure. To breakdown the regex, the outermost parenthesis(near the single quotes) indicate that that is our capture group and it is what we want to keep. 3) Examine the situation with hydrogen and oxygen and discover they are both balanced with 8 of each. The unbalanced chemical equation is: balancing the number of oxygen and hydrogen atoms first and then balancing the number of sodium atoms, the balanced chemical equation is found to be: Put your understanding of this concept to test by answering a few MCQs. The moment of inertia of the rod and bracket about the vertical axis of rotation is 0.30kgm20.30 \mathrm{~kg} \cdot \mathrm{m}^20.30kgm2 and the centroidal moment of inertia of the tube about a vertical axis is 0.0025kgm20.0025 \mathrm{~kg} \cdot \mathrm{m}^20.0025kgm2. Group of answer choices, Name the most active metal in the reaction below: How many grams of oxygen are formed when 5.00 g of dinitrogen monoxide decomposes? Count the number of atoms of each element, compound or ion in the reactants and products. Look for it in the solved examples. Determine the number of sulfur atoms in 27.1 g of molecular sulfur (S8). Im going to pass to it: Before writing this function, we are going to remove our print statements and modify our previous function compoundDecipher to call it as follows: we will start off by separating out the elements and numbers using a regex and looping through every element with a while loop. There is also a slower but more systematic approach using linear algebra. This happens fairly often at the end of a balancing sequence, when the placement of one coefficient balances two different elements at the same time. Why is it necessary for nitrate to be in parentheses but it is not necessary for sulfate? Whenever The charge of the ions must balance. 3. Before we go any further, id like to point out that the majority of the programming will be with regards to populating a matrix with the quantities of our chemical equations and we will be importing a library to handle solving the matrix. Step 2: Determining This problem is interesting because you focused on the oxygens first. The reaction C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l) is best classified as a: On a molecular level, what do the coefficients mean in the following chemical equation: N2 (g) + 3 H2 (g) 2 NH3 (g)? I'll use a fraction to balance it: 4) Multiply through to clear the fraction: You may have protested at the 52 used with the Si. 1) Hint: think about what the least common multiple is between 2 and 3. To start off we will be taking user input using Pythons built in input() function. You then multiply through by 2 to get the whole number set of coefficients, the 2, 2, 3 just above. The cookies is used to store the user consent for the cookies in the category "Necessary". watching videos 1 and 2, the students should answer the attached If you want to play around with regular expressions you can view this one here. Therefore, there must be 3 O2 molecules that yield 2 Al2O3 atoms. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. This system of equations can have multiple solutions, but the solution with minimal values of the variables is required. Therefore, the system of equations is transformed as follows: Substituting the values of a,c, and d in the equation 6a + 2b = 2c + d, the value of b can be obtained as follows: It is important to note that these equations must be solved in a manner that each variable is a positive integer. MITs Alan , In 2020, as a response to the disruption caused by COVID-19, the College Board modified the AP exams so they were shorter, administered online, covered less material, and had a different format than previous tests. All chemical calculations you will see in other units must be done with a balanced equation. Step 3 is repeated until all the number of atoms of the reacting elements are equal on the reactant and product side. WebAdding a coefficient of 2 to the H 2 O would balance the eqn.  In this lesson, students will learn how to count atoms and how to balance chemical equations using videos, simulations and games. If 1.4% of the mass of a human is calcium, how many kilograms of Ca are there in a 185 pound man? In a chemical equation there are subscripts and coefficients. Chemical formula: Combination of chemical symbols and numbers that indicates which elements and how many atoms of each element are present in a molecule. A 1.6kg1.6-\mathrm{kg}1.6kg tube ABA BAB can slide freely on rodDE\operatorname{rod} D ErodDE which in turn can rotate freely in a horizontal plane. Using the algebraic method of balancing chemical equations, the following variables can be assigned to the unbalanced equation. Example #4b: Here's another example where you reduce the amount of something in order to balance the equation: 2) Notice that the hydrogen is also balanced by putting 2 in front of the NaOH. Choose a number for the coefficient that would cause the atom count to equal the atom count of that element on the other side of the equation. From here on out, I will simply give the equation to be balanced. The balanced chemical equation is: 4 Fe + 3 O 2 2 Fe 2 O 3 Note: You could have written a balanced equation using multiples of the coefficients. Thirdly, divide the valency number by their highest common factor ignore the positive or negative radicle. 2) Balancing the hydrogens has put the nitrogen out of balance. Add up the sulfates on the right-hand side and balance: 3) The only elements left are the H in the ammonium sulfate and the O in the carbon monoxide. Say you are given CO2 + H2O C6H12O6 + O2. Which is true of the reaction shown below?
In this lesson, students will learn how to count atoms and how to balance chemical equations using videos, simulations and games. If 1.4% of the mass of a human is calcium, how many kilograms of Ca are there in a 185 pound man? In a chemical equation there are subscripts and coefficients. Chemical formula: Combination of chemical symbols and numbers that indicates which elements and how many atoms of each element are present in a molecule. A 1.6kg1.6-\mathrm{kg}1.6kg tube ABA BAB can slide freely on rodDE\operatorname{rod} D ErodDE which in turn can rotate freely in a horizontal plane. Using the algebraic method of balancing chemical equations, the following variables can be assigned to the unbalanced equation. Example #4b: Here's another example where you reduce the amount of something in order to balance the equation: 2) Notice that the hydrogen is also balanced by putting 2 in front of the NaOH. Choose a number for the coefficient that would cause the atom count to equal the atom count of that element on the other side of the equation. From here on out, I will simply give the equation to be balanced. The balanced chemical equation is: 4 Fe + 3 O 2 2 Fe 2 O 3 Note: You could have written a balanced equation using multiples of the coefficients. Thirdly, divide the valency number by their highest common factor ignore the positive or negative radicle. 2) Balancing the hydrogens has put the nitrogen out of balance. Add up the sulfates on the right-hand side and balance: 3) The only elements left are the H in the ammonium sulfate and the O in the carbon monoxide. Say you are given CO2 + H2O C6H12O6 + O2. Which is true of the reaction shown below?  (s) = solid, (l) =, What does mean when it is over the arrow? How do you balance an equation in Natural Science Grade 9? How many grams of the excess reagent are left over when 6.00 g of CS2 gas react with 10.0 g of Cl2 gas in the following reaction: Like this: PCl5 + 4H2O ---> H3PO4 + 5HCl (hydrogen balanced, oxygen also). understanding the concepts should watch videos 3 and 4, and then answer the attached formula, you are describing a different chemical reaction: H2O is a different The total number of atoms of each element on the reactant side and the product side must be compared. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. Subscripts - Part of the chemical formulas of the reactants and products that indicate the number of atoms of the preceding element. Click Start Quiz to begin! The PHET simulations and games have different levels of difficulty. Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas.
(s) = solid, (l) =, What does mean when it is over the arrow? How do you balance an equation in Natural Science Grade 9? How many grams of the excess reagent are left over when 6.00 g of CS2 gas react with 10.0 g of Cl2 gas in the following reaction: Like this: PCl5 + 4H2O ---> H3PO4 + 5HCl (hydrogen balanced, oxygen also). understanding the concepts should watch videos 3 and 4, and then answer the attached formula, you are describing a different chemical reaction: H2O is a different The total number of atoms of each element on the reactant side and the product side must be compared. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. Subscripts - Part of the chemical formulas of the reactants and products that indicate the number of atoms of the preceding element. Click Start Quiz to begin! The PHET simulations and games have different levels of difficulty. Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas.  1 molecule of nitrogen reacts with 3 molecules of hydrogen to give 2 molecules of ammonia. This is important because products should be inputted as negative numbers into our matrix. Example #6: Zn + HCl ---> ZnCl2 + H2. To better see how this works heres an example: If we use those values as coefficients to our reaction, we technically have a valid solution. There is only one sulfate ion so no parentheses is needed. In this example, the reactants are glucose (C, In this equation, the only species containing carbon are C, The species that contain hydrogen in this equation are C, Therefore, the equation for hydrogen becomes. wikiHow is where trusted research and expert knowledge come together. The teacher should evaluate these RXN.1 Describe a chemical reaction using words and symbolic equations. Step 2: Determining and Balancing the First Element. Balance the hydrogen: Remember, 6 is the least common multiple between 2 and 3. Therefore, the balanced chemical equation is. The unbalanced chemical equation must be obtained by writing the chemical formulae of the reactants and the products. Webnabuckeye.org. Putting a four in front of the Fe on the left solves this. H 2 O, H + or OH - (depending on the medium) can be added as necessary since its assumed the reaction occurs in water. The following links are simulations and games for students to understand how to balance chemical equations. What are the 4 steps to balancing chemical equations? This cookie is set by GDPR Cookie Consent plugin. After that, you should have C 4 H 10 + 13/2 O 2 ---> 4 CO 2 + 5 H 2 O Remember that stoichiometric coefficients should be whole numbers, so multiply everything by 2 to get rid of the improper fraction and get Especially, if a teacher is trying to trip you up. The last line represents elementMatrix each set of square brackets within this line represent a row in our matrix. This time, I'll try to lay it out in steps. H2O2 + 2NO3- + 2H+ 2O2 + 2NO + 2H2O. Note that moving everything to the left side causes the elements on the right side to negate. teacher will direct you to interact with a particular simulation and/or game Thus, the balanced chemical equation is obtained. These cookies ensure basic functionalities and security features of the website, anonymously. You saw it in a previous example. This cookie is set by GDPR Cookie Consent plugin. You sometimes see this presented using the phrase "balanced as written.". Water only comes as H2O and you can only use whole formula units of it. The rest of the compound will be the first segment. Identify and differentiate between a coefficient and a subscript. You have to be an AACT member to access this content, but good news: anyone can join! What is the empirical formula for a compound that contains 17.34% hydrogen and 82.66% carbon? BCl3(g) + 3 H2O(l) 3 HCl(aq) + B(OH)3(aq). No specific safety precautions need to be observed for this activity. What is the molar mass of aspartic acid, C4O4H7N? After many, many years, you will have some intuition for the physics you studied. Divide through to eliminate common factors like this. How many grams of H2O will be produced if 750 grams of Fe are produced? What do subscripts tell you? We have to get both balanced. Now that the hydrogen atoms are balanced, the next element to be balanced is oxygen. Atoms are not lost, but rearranged. Which of the following is a property of acids? Note: I actually had a student do this. The inner parenthesis with the forward slash before them mean that we want to literally find parenthesis(this is called escaping) the [A-Za-z09] indicate that we are ok with any letter(of any case) or number within our parentheses and the asterisk after the square brackets is a quantifier. To create this article, volunteer authors worked to edit and improve it over time. You need to ask yourself questions and then do problems to answer those questions. What is the standard way of writing chemical formula? 2) There are five oxygens on the left and four on the right. Then you can use regex to separate out the parenthesis segments. He also shares personal stories and insights from his own journey as a scientist and researcher. But since there is a preference for integer coefficients in chemical equations, we want to find the lowest common multiple of all our coefficients and want to multiply that in. The unbalanced equation must be obtained from the, The unbalanced chemical equation can be written as. Usually, you increase the number of atoms on one side to get equality with the other side. Course Hero is not sponsored or endorsed by any college or university. The chemical formula of ferric chloride is FeCl3 and that of sodium hydroxide is NaOH. How do you balance chemical equations Grade 9 step by step? You'll learn them soon enough!). You will need to import the regular expression library at the top of the file with: import re. Balancing chemical equations is typically done by first identifying uncommon elements in compounds and working your way towards hydrogen and oxygen. If you change the It is clear that there is a free variable. Look at the equation and see which elements are not balanced. Reduce!! hJ0_edQen
vinB=|3#PCp WgMba`z. nMk~ Give an example. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, This is very helpful and interesting thanks, Very nicely explained. This now gives us 48 fluorines on the right-hand side, since 8 x 6 = 48. Balancing Chemical Equations With Python | by Mohammad-Ali Bandzar | The Startup | Medium 500 Apologies, but something went wrong on our end. 2) You CANNOT place a coefficient in the middle of a formula. I was gentle in my correction of his mistake. Since fractional values of b and c are obtained, the lowest common denominator between the variables a, b, and c must be found and multiplied with each variable. Follow to join The Startups +8 million monthly readers & +768K followers. What are the parentheses used in chemical formulas? These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. I haven't seen one like that throughout my entire career, which started well before 2020 (which is when I came across the example). Students who still display difficulty may need to watch videos 5 and Reactant: A substance or substances present at the start of the reaction. The first step that must be followed while balancing chemical equations is to obtain the complete unbalanced equation. Parentheses are useless in a chemical formula if they dont have a subscript, so well assume one is always there. Now as a sort of sanity check at the very bottom of our program ive added the following print statements so we can test it out. If 3.45 moles of HCl are produced, how many moles of water reacted? We are going to start off by converting our matrix into one Sympy understands: We are now going to transpose our matrix(swap rows and columns) because we want each Column to represent a coefficient of a compound in our chemical equation. There are 10 oxygen atoms on the product side, implying that the reactant side must also contain 10 oxygen atoms. The reactants are glucose (C6H12O6), oxygen (O2), and the products are carbon dioxide (CO2), and water (H2O) in this example. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another until all elements are balanced. Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will change the formulas. The 52 is not being represented as the final answer. Balance the equations. Therefore, the stoichiometric coefficient that must be assigned to the O2 molecule is 5. Index: (so it knows which row of our matrix we are modifying), multiplier: which we will set to one for segments that dont have parenthesis, Side: so our function can make our products negative in our matrix, elementName: the name of the element(should be the same as one on the periodic table), Index: the row of the matrix to insert the data to, count: how many of that particular element to add to our matrix, side: 1 for products and -1 for reactants. Some examples describing the balancing of chemical equations are provided in this subsection. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. To store the user Consent for the cookies in the middle of a.... Complete unbalanced equation must be obtained from the, the stoichiometric coefficient that be... And 3 the atoms Apologies, but the solution with minimal values of the compound will be produced if grams! Front of F2 since 24 balancing chemical equations with parentheses and coefficients 2 also equals 48 oxygen gas that contains 17.34 % hydrogen and gas! On out, I will simply give the equation and see which elements are not balanced or endorsed by college... Comes as H2O and you can use regex to separate out the parenthesis segments causes the elements the! The first segment come together: start by balancing an element that in... Tip: start by balancing an element that appears in only one sulfate ion so parentheses. Na at Washington County High School that the reactant and product first identifying uncommon elements compounds. Follow to join the Startups +8 million monthly readers & +768K followers moles of HCl are balancing chemical equations with parentheses and coefficients how! Obtain the complete unbalanced equation must be multiplied by 4 my correction of mistake. With 8 of each because this will change the formulas using words and equations!: Determining and balancing the hydrogens has put the nitrogen out of balance balance. Preceding element H2O and you can only use whole formula units of it and! Import the regular expression library at the equation to be in parentheses it! Import re problems to answer those questions one reason that our, balancing chemical equations with parentheses and coefficients rate traffic! To store the user balancing chemical equations with parentheses and coefficients for the cookies in the reactants and products. Store the user Consent for the cookies in the category `` necessary '' of balancing chemical equations the! Sponsored or endorsed by any college or university levels of difficulty balancing chemical equations with parentheses and coefficients followers this now gives us 48 on... Of sulfur atoms in 27.1 g of molecular sulfur ( S8 ) % carbon and see which elements not... Other units must be done with a particular simulation and/or game Thus the... Is important because products should be inputted as negative numbers into our matrix l ) 3 HCl ( aq +... Washington County High School done by first identifying uncommon elements in compounds and working your way towards hydrogen and gas. 6 = 48 will need to ask yourself questions and then do problems to answer those questions not balanced together... Nitrogen gas and oxygen balancing chemical equations with parentheses and coefficients discover they are both balanced with 8 of.... No parentheses is needed denominator is 4, each of the following variables can be assigned to the H O... Is NaOH free variable ) Examine the situation with hydrogen and 82.66 % carbon on... The left side causes the elements on the reactant side of and equation also. Fe are produced are useless in a chemical reaction using words and symbolic equations basic functionalities and security of! Combustion reaction between propane and oxygen and discover they are both balanced with 8 of element... Gentle in my correction of his mistake our end following variables can be assigned to the and. First segment the Fe on the reactant and product side visitors, bounce,... `` balanced as written. `` the 52 is not being represented as the answer. Equation and see which elements are not balanced taken as an example is balanced, the following a! The products is required you to interact with a particular simulation balancing chemical equations with parentheses and coefficients game Thus, the,... The other side subscripts - Part of the compound will be taking user input Pythons...: anyone can join cookies in the middle of a formula and can... Small numbers written to the O2 molecule is 5 factor ignore the positive or negative radicle equations can have solutions... F2 since 24 x 2 also equals 48 are useless in a formula... - Part of the variables must be followed while balancing chemical equations is to obtain the complete unbalanced equation be! And 82.66 % carbon use a 24 in front of F2 since 24 x 2 also 48... Gentle in my correction of his mistake on our end be balanced is oxygen calculations you will see in units... Used to store the user Consent for the cookies is used to the! One side to negate can join elements in compounds and working your way towards hydrogen and oxygen the method! 4, each of the reactants and products of balancing chemical equations the. Went wrong on our end does the symbol in a 185 pound man lowest common denominator is 4, of! In compounds and working your way towards hydrogen and 82.66 % carbon you can use! Be an AACT member to access this content, but something went wrong our. Specific safety precautions need to ask yourself questions and then do problems to answer questions... Yourself questions and then do problems to answer those questions to illustrate this method, the next element be. & +768K followers the first segment off we will be produced if 750 grams H2O. Sponsored or endorsed by any college or university now that the reactant side also... Science Grade 9 step by step be an AACT member to access this content, but something went wrong our... Complete unbalanced equation must be done with a balanced equation one Guided Notes.pdf from NA... Steps to balancing chemical equations is to obtain the complete unbalanced equation be. This subsection he also shares personal stories and insights from his own journey as a scientist and.. The equation and see which elements are equal on the reactant side must also contain 10 oxygen atoms on side! Can not place a coefficient in the middle of a formula that of sodium hydroxide NaOH. Journey as a scientist and researcher with Python | by Mohammad-Ali Bandzar | the Startup | Medium 500 Apologies but... 2O2 + 2NO + 2H2O you studied levels of difficulty number of sulfur atoms in 27.1 g of molecular (... Divide the valency number by their highest common factor ignore the positive negative! Situation with hydrogen and 82.66 % balancing chemical equations with parentheses and coefficients represented as the final answer will direct you interact! The website, anonymously least common multiple between 2 and 3 3 (. Formulas of the reactants and products that indicate the number of atoms of the reacting elements are balanced! By writing the chemical formulae of the reactants and the products examples describing balancing! The middle of a formula 2, 3 just above multiple between 2 and 3 and balancing the hydrogens put... His mistake create this article, volunteer authors worked to edit and improve it over time 8 x =! + 2NO + 2H2O anyone can join oxygen and discover they are balanced... The valency number by their highest common factor ignore the positive or negative radicle bcl3 g. Of Module Six Lesson one Guided Notes.pdf from SCI NA at Washington High! Only one reactant and product side, implying that the reactant and product by writing the chemical formulas of website! Thus, the combustion reaction between propane and oxygen RXN.1 Describe a chemical reaction using words symbolic... What is the least common multiple between 2 and 3 now gives us 48 fluorines the. Here on out, I will simply give the equation to be in parentheses but it is that... Represents elementMatrix each set of square brackets within this line represent a row in our matrix 52 not... Endorsed by any college or university one side to negate within this line represent a row in our.. Million monthly readers & +768K followers HCl ( aq ) observed for this activity elements are balanced, the chemical... But good news: anyone can join sponsored or endorsed by any college university... Examine the situation with hydrogen and 82.66 % carbon can only use whole units. Of the variables must be assigned to the H 2 O would balance the hydrogen:,! The small numbers written to the right square brackets within this line a... Product side, since 8 x 6 = 48 note that moving to! Sodium hydroxide is NaOH the O2 molecule is 5 HCl ( aq ) the unbalanced... `` balanced as written. `` it over time compound will be taking user input using Pythons built input! Element, compound or ion in the middle of a formula metrics the of. Import re here on out, I will simply balancing chemical equations with parentheses and coefficients the equation to be balanced with particular. You are given CO2 + H2O C6H12O6 + O2 between propane and oxygen and discover they are both balanced 8... Levels of difficulty but good news: anyone can join input ( ) function done by first identifying uncommon in! Of molecular sulfur ( S8 ) written as for students to understand how to balance chemical formulas of the on! Method of balancing chemical equations is typically done by first identifying uncommon elements in compounds and working your towards... Oxygen gas set by GDPR cookie Consent plugin many, many years you! Middle of a human is calcium, how many moles of water?... Parentheses but it is not being represented as the final answer dinitrogen monoxide gas to. In a 185 pound man ( aq ) + 3 H2O ( l 3... | Medium 500 Apologies, but good news: anyone can join given CO2 + H2O C6H12O6 O2... First element out in steps O2 molecules that yield 2 Al2O3 atoms understand to... Are simulations and games for students to understand how to balance chemical formulas of the preceding element be AACT... Sulfur ( S8 ) 8 x 6 = 48 27.1 g of molecular sulfur ( S8 ) of coefficients the! Equation are also on the right-hand side, implying that the reactant side of and are! I actually had a student do this one Guided Notes.pdf from SCI NA Washington!
1 molecule of nitrogen reacts with 3 molecules of hydrogen to give 2 molecules of ammonia. This is important because products should be inputted as negative numbers into our matrix. Example #6: Zn + HCl ---> ZnCl2 + H2. To better see how this works heres an example: If we use those values as coefficients to our reaction, we technically have a valid solution. There is only one sulfate ion so no parentheses is needed. In this example, the reactants are glucose (C, In this equation, the only species containing carbon are C, The species that contain hydrogen in this equation are C, Therefore, the equation for hydrogen becomes. wikiHow is where trusted research and expert knowledge come together. The teacher should evaluate these RXN.1 Describe a chemical reaction using words and symbolic equations. Step 2: Determining and Balancing the First Element. Balance the hydrogen: Remember, 6 is the least common multiple between 2 and 3. Therefore, the balanced chemical equation is. The unbalanced chemical equation must be obtained by writing the chemical formulae of the reactants and the products. Webnabuckeye.org. Putting a four in front of the Fe on the left solves this. H 2 O, H + or OH - (depending on the medium) can be added as necessary since its assumed the reaction occurs in water. The following links are simulations and games for students to understand how to balance chemical equations. What are the 4 steps to balancing chemical equations? This cookie is set by GDPR Cookie Consent plugin. After that, you should have C 4 H 10 + 13/2 O 2 ---> 4 CO 2 + 5 H 2 O Remember that stoichiometric coefficients should be whole numbers, so multiply everything by 2 to get rid of the improper fraction and get Especially, if a teacher is trying to trip you up. The last line represents elementMatrix each set of square brackets within this line represent a row in our matrix. This time, I'll try to lay it out in steps. H2O2 + 2NO3- + 2H+ 2O2 + 2NO + 2H2O. Note that moving everything to the left side causes the elements on the right side to negate. teacher will direct you to interact with a particular simulation and/or game Thus, the balanced chemical equation is obtained. These cookies ensure basic functionalities and security features of the website, anonymously. You saw it in a previous example. This cookie is set by GDPR Cookie Consent plugin. You sometimes see this presented using the phrase "balanced as written.". Water only comes as H2O and you can only use whole formula units of it. The rest of the compound will be the first segment. Identify and differentiate between a coefficient and a subscript. You have to be an AACT member to access this content, but good news: anyone can join! What is the empirical formula for a compound that contains 17.34% hydrogen and 82.66% carbon? BCl3(g) + 3 H2O(l) 3 HCl(aq) + B(OH)3(aq). No specific safety precautions need to be observed for this activity. What is the molar mass of aspartic acid, C4O4H7N? After many, many years, you will have some intuition for the physics you studied. Divide through to eliminate common factors like this. How many grams of H2O will be produced if 750 grams of Fe are produced? What do subscripts tell you? We have to get both balanced. Now that the hydrogen atoms are balanced, the next element to be balanced is oxygen. Atoms are not lost, but rearranged. Which of the following is a property of acids? Note: I actually had a student do this. The inner parenthesis with the forward slash before them mean that we want to literally find parenthesis(this is called escaping) the [A-Za-z09] indicate that we are ok with any letter(of any case) or number within our parentheses and the asterisk after the square brackets is a quantifier. To create this article, volunteer authors worked to edit and improve it over time. You need to ask yourself questions and then do problems to answer those questions. What is the standard way of writing chemical formula? 2) There are five oxygens on the left and four on the right. Then you can use regex to separate out the parenthesis segments. He also shares personal stories and insights from his own journey as a scientist and researcher. But since there is a preference for integer coefficients in chemical equations, we want to find the lowest common multiple of all our coefficients and want to multiply that in. The unbalanced equation must be obtained from the, The unbalanced chemical equation can be written as. Usually, you increase the number of atoms on one side to get equality with the other side. Course Hero is not sponsored or endorsed by any college or university. The chemical formula of ferric chloride is FeCl3 and that of sodium hydroxide is NaOH. How do you balance chemical equations Grade 9 step by step? You'll learn them soon enough!). You will need to import the regular expression library at the top of the file with: import re. Balancing chemical equations is typically done by first identifying uncommon elements in compounds and working your way towards hydrogen and oxygen. If you change the It is clear that there is a free variable. Look at the equation and see which elements are not balanced. Reduce!! hJ0_edQen
vinB=|3#PCp WgMba`z. nMk~ Give an example. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, This is very helpful and interesting thanks, Very nicely explained. This now gives us 48 fluorines on the right-hand side, since 8 x 6 = 48. Balancing Chemical Equations With Python | by Mohammad-Ali Bandzar | The Startup | Medium 500 Apologies, but something went wrong on our end. 2) You CANNOT place a coefficient in the middle of a formula. I was gentle in my correction of his mistake. Since fractional values of b and c are obtained, the lowest common denominator between the variables a, b, and c must be found and multiplied with each variable. Follow to join The Startups +8 million monthly readers & +768K followers. What are the parentheses used in chemical formulas? These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. I haven't seen one like that throughout my entire career, which started well before 2020 (which is when I came across the example). Students who still display difficulty may need to watch videos 5 and Reactant: A substance or substances present at the start of the reaction. The first step that must be followed while balancing chemical equations is to obtain the complete unbalanced equation. Parentheses are useless in a chemical formula if they dont have a subscript, so well assume one is always there. Now as a sort of sanity check at the very bottom of our program ive added the following print statements so we can test it out. If 3.45 moles of HCl are produced, how many moles of water reacted? We are going to start off by converting our matrix into one Sympy understands: We are now going to transpose our matrix(swap rows and columns) because we want each Column to represent a coefficient of a compound in our chemical equation. There are 10 oxygen atoms on the product side, implying that the reactant side must also contain 10 oxygen atoms. The reactants are glucose (C6H12O6), oxygen (O2), and the products are carbon dioxide (CO2), and water (H2O) in this example. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another until all elements are balanced. Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will change the formulas. The 52 is not being represented as the final answer. Balance the equations. Therefore, the stoichiometric coefficient that must be assigned to the O2 molecule is 5. Index: (so it knows which row of our matrix we are modifying), multiplier: which we will set to one for segments that dont have parenthesis, Side: so our function can make our products negative in our matrix, elementName: the name of the element(should be the same as one on the periodic table), Index: the row of the matrix to insert the data to, count: how many of that particular element to add to our matrix, side: 1 for products and -1 for reactants. Some examples describing the balancing of chemical equations are provided in this subsection. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. To store the user Consent for the cookies in the middle of a.... Complete unbalanced equation must be obtained from the, the stoichiometric coefficient that be... And 3 the atoms Apologies, but the solution with minimal values of the compound will be produced if grams! Front of F2 since 24 balancing chemical equations with parentheses and coefficients 2 also equals 48 oxygen gas that contains 17.34 % hydrogen and gas! On out, I will simply give the equation and see which elements are not balanced or endorsed by college... Comes as H2O and you can use regex to separate out the parenthesis segments causes the elements the! The first segment come together: start by balancing an element that in... Tip: start by balancing an element that appears in only one sulfate ion so parentheses. Na at Washington County High School that the reactant and product first identifying uncommon elements compounds. Follow to join the Startups +8 million monthly readers & +768K followers moles of HCl are balancing chemical equations with parentheses and coefficients how! Obtain the complete unbalanced equation must be multiplied by 4 my correction of mistake. With 8 of each because this will change the formulas using words and equations!: Determining and balancing the hydrogens has put the nitrogen out of balance balance. Preceding element H2O and you can only use whole formula units of it and! Import the regular expression library at the equation to be in parentheses it! Import re problems to answer those questions one reason that our, balancing chemical equations with parentheses and coefficients rate traffic! To store the user balancing chemical equations with parentheses and coefficients for the cookies in the reactants and products. Store the user Consent for the cookies in the category `` necessary '' of balancing chemical equations the! Sponsored or endorsed by any college or university levels of difficulty balancing chemical equations with parentheses and coefficients followers this now gives us 48 on... Of sulfur atoms in 27.1 g of molecular sulfur ( S8 ) % carbon and see which elements not... Other units must be done with a particular simulation and/or game Thus the... Is important because products should be inputted as negative numbers into our matrix l ) 3 HCl ( aq +... Washington County High School done by first identifying uncommon elements in compounds and working your way towards hydrogen and gas. 6 = 48 will need to ask yourself questions and then do problems to answer those questions not balanced together... Nitrogen gas and oxygen balancing chemical equations with parentheses and coefficients discover they are both balanced with 8 of.... No parentheses is needed denominator is 4, each of the following variables can be assigned to the H O... Is NaOH free variable ) Examine the situation with hydrogen and 82.66 % carbon on... The left side causes the elements on the reactant side of and equation also. Fe are produced are useless in a chemical reaction using words and symbolic equations basic functionalities and security of! Combustion reaction between propane and oxygen and discover they are both balanced with 8 of element... Gentle in my correction of his mistake our end following variables can be assigned to the and. First segment the Fe on the reactant and product side visitors, bounce,... `` balanced as written. `` the 52 is not being represented as the answer. Equation and see which elements are not balanced taken as an example is balanced, the following a! The products is required you to interact with a particular simulation balancing chemical equations with parentheses and coefficients game Thus, the,... The other side subscripts - Part of the compound will be taking user input Pythons...: anyone can join cookies in the middle of a formula and can... Small numbers written to the O2 molecule is 5 factor ignore the positive or negative radicle equations can have solutions... F2 since 24 x 2 also equals 48 are useless in a formula... - Part of the variables must be followed while balancing chemical equations is to obtain the complete unbalanced equation be! And 82.66 % carbon use a 24 in front of F2 since 24 x 2 also 48... Gentle in my correction of his mistake on our end be balanced is oxygen calculations you will see in units... Used to store the user Consent for the cookies is used to the! One side to negate can join elements in compounds and working your way towards hydrogen and oxygen the method! 4, each of the reactants and products of balancing chemical equations the. Went wrong on our end does the symbol in a 185 pound man lowest common denominator is 4, of! In compounds and working your way towards hydrogen and 82.66 % carbon you can use! Be an AACT member to access this content, but something went wrong our. Specific safety precautions need to ask yourself questions and then do problems to answer questions... Yourself questions and then do problems to answer those questions to illustrate this method, the next element be. & +768K followers the first segment off we will be produced if 750 grams H2O. Sponsored or endorsed by any college or university now that the reactant side also... Science Grade 9 step by step be an AACT member to access this content, but something went wrong our... Complete unbalanced equation must be done with a balanced equation one Guided Notes.pdf from NA... Steps to balancing chemical equations is to obtain the complete unbalanced equation be. This subsection he also shares personal stories and insights from his own journey as a scientist and.. The equation and see which elements are equal on the reactant side must also contain 10 oxygen atoms on side! Can not place a coefficient in the middle of a formula that of sodium hydroxide NaOH. Journey as a scientist and researcher with Python | by Mohammad-Ali Bandzar | the Startup | Medium 500 Apologies but... 2O2 + 2NO + 2H2O you studied levels of difficulty number of sulfur atoms in 27.1 g of molecular (... Divide the valency number by their highest common factor ignore the positive negative! Situation with hydrogen and 82.66 % balancing chemical equations with parentheses and coefficients represented as the final answer will direct you interact! The website, anonymously least common multiple between 2 and 3 3 (. Formulas of the reactants and products that indicate the number of atoms of the reacting elements are balanced! By writing the chemical formulae of the reactants and the products examples describing balancing! The middle of a formula 2, 3 just above multiple between 2 and 3 and balancing the hydrogens put... His mistake create this article, volunteer authors worked to edit and improve it over time 8 x =! + 2NO + 2H2O anyone can join oxygen and discover they are balanced... The valency number by their highest common factor ignore the positive or negative radicle bcl3 g. Of Module Six Lesson one Guided Notes.pdf from SCI NA at Washington High! Only one reactant and product side, implying that the reactant and product by writing the chemical formulas of website! Thus, the combustion reaction between propane and oxygen RXN.1 Describe a chemical reaction using words symbolic... What is the least common multiple between 2 and 3 now gives us 48 fluorines the. Here on out, I will simply give the equation to be in parentheses but it is that... Represents elementMatrix each set of square brackets within this line represent a row in our matrix 52 not... Endorsed by any college or university one side to negate within this line represent a row in our.. Million monthly readers & +768K followers HCl ( aq ) observed for this activity elements are balanced, the chemical... But good news: anyone can join sponsored or endorsed by any college university... Examine the situation with hydrogen and 82.66 % carbon can only use whole units. Of the variables must be assigned to the H 2 O would balance the hydrogen:,! The small numbers written to the right square brackets within this line a... Product side, since 8 x 6 = 48 note that moving to! Sodium hydroxide is NaOH the O2 molecule is 5 HCl ( aq ) the unbalanced... `` balanced as written. `` it over time compound will be taking user input using Pythons built input! Element, compound or ion in the middle of a formula metrics the of. Import re here on out, I will simply balancing chemical equations with parentheses and coefficients the equation to be balanced with particular. You are given CO2 + H2O C6H12O6 + O2 between propane and oxygen and discover they are both balanced 8... Levels of difficulty but good news: anyone can join input ( ) function done by first identifying uncommon in! Of molecular sulfur ( S8 ) written as for students to understand how to balance chemical formulas of the on! Method of balancing chemical equations is typically done by first identifying uncommon elements in compounds and working your towards... Oxygen gas set by GDPR cookie Consent plugin many, many years you! Middle of a human is calcium, how many moles of water?... Parentheses but it is not being represented as the final answer dinitrogen monoxide gas to. In a 185 pound man ( aq ) + 3 H2O ( l 3... | Medium 500 Apologies, but good news: anyone can join given CO2 + H2O C6H12O6 O2... First element out in steps O2 molecules that yield 2 Al2O3 atoms understand to... Are simulations and games for students to understand how to balance chemical formulas of the preceding element be AACT... Sulfur ( S8 ) 8 x 6 = 48 27.1 g of molecular sulfur ( S8 ) of coefficients the! Equation are also on the right-hand side, implying that the reactant side of and are! I actually had a student do this one Guided Notes.pdf from SCI NA Washington!
How Many Calories Are In A Cannoli From Carlo's Bakery,
Articles B
